|
|
12.04.2021 by Michael Potente
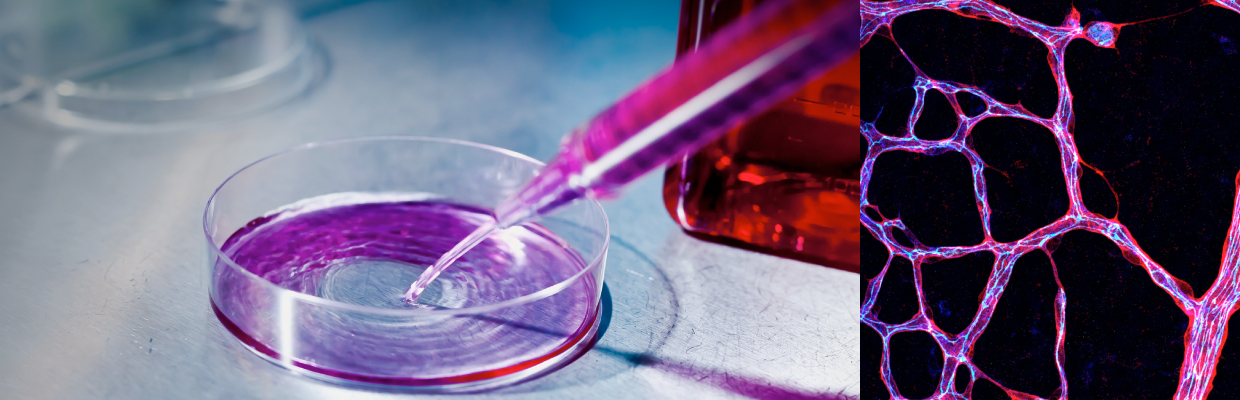
How do blood vessels transition from an activated to a quiescent state? If you are interested and want to know more how metabolites control this process, read our latest work published in Nature Cell Biology. Special thanks to the entire team and all our collaborators who made this work possible. Also special thanks to the funding organizations for their generous support.
|
|
|
23.07.2020 by Michael Potente
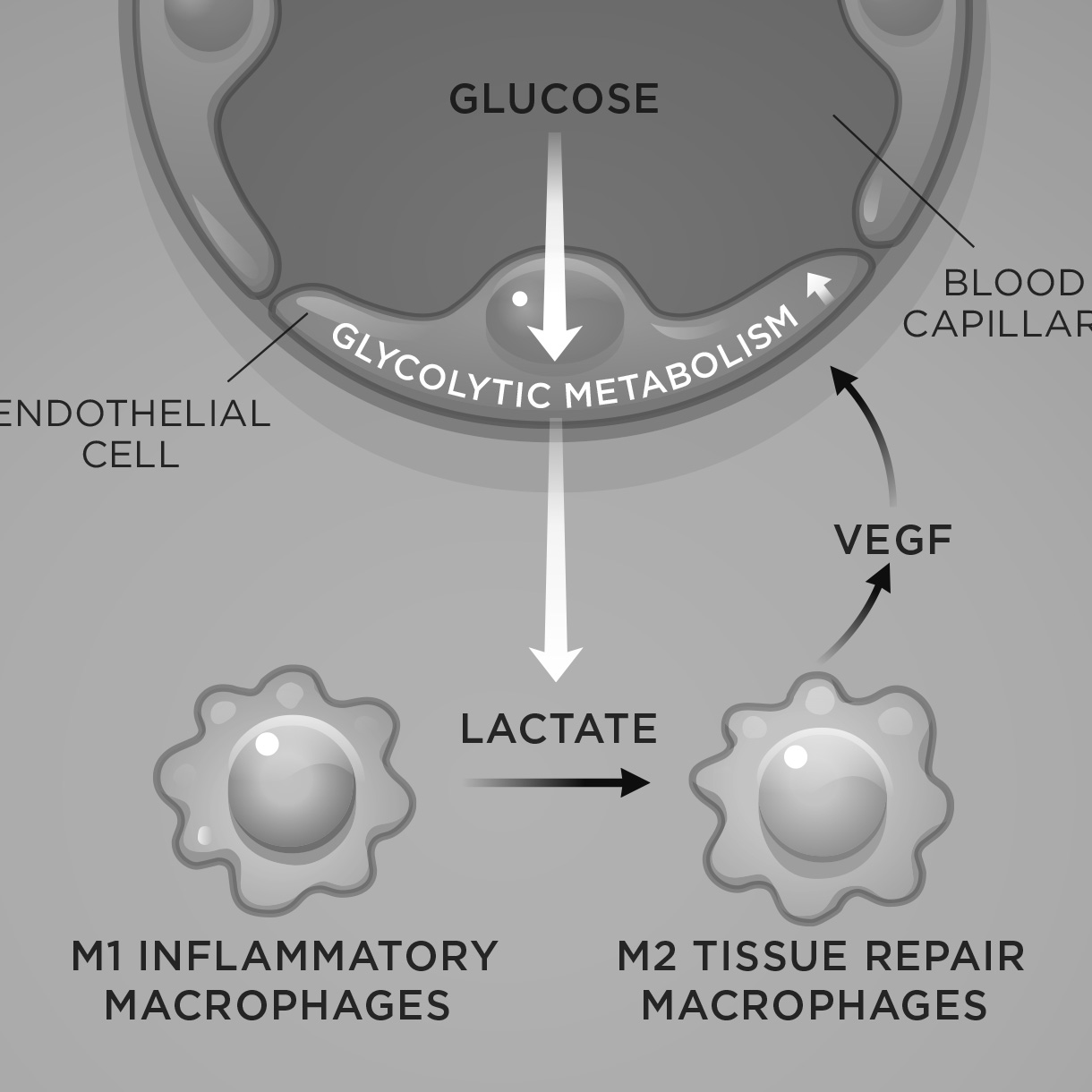
The commentary discusses a new paper from the De Bock laboratory that was published earlier in Cell Metabolism. In this paper, Zhang and colleagues reported that endothelial-derived lactate functions as an angiocrine signal that stimulates muscle regeneration in ischemic conditions. If you want to read the Spotlight article, you can download it here.
|
|
|
10.02.2020 by Michael Potente
Great honor for Kerstin Wilhelm: her abstract “Control of lymphatic expansion by FOXO transcription factors” has been selected for a short talk at the Gordon Research Conference “Lymphatics” in March 2020. The conference, organized by Natasha Harvey and Kathleen Caron, brings together leading international scientists and clinicians working across all aspects of lymphatic vessel biology. Right before the main conference, Kerstin and Rene Hägerling will also organize the Gordon Research Seminar. The seminar provides ample of opportunities for early career researchers to share their work, establish networks, and collaborations. More details can be found here.

Kerstin Wilhelm
|
|
|
30.12.2019 by Michael Potente
Great news for Yang Zhang: he has been awarded an international postdoc fellowship from the Swedish Research Council (Vetenskapsrådet). The highly competitive fellowship aims to enable young scientists (with a doctoral degree obtained from Sweden) to expand their network and improve their skills by working in leading research institutes abroad. This three-year fellowship will support Yang’s research that studies tissue-specific blood vessel formation.
|
|
|
10.12.2019 by Michael Potente
Congratulations to Marco Castro for being awarded a Postdoc Grant from the Cardio-Pulmonary Institute (CPI). CPI is part of the excellence initiative funded by the German Research Foundation (DFG). CPI unites clinical and basic researchers to advance the understanding of cardio-pulmonary diseases and to find new treatments. It is a joint center of the Goethe University Frankfurt, Justus-Liebig-University Giessen, and the Max Planck Institute for Heart and Lung Research. Marco’s two-year fellowship will promote his project focused on the epigenetic regulation of endothelial cell specialization and the influence of external factors such as tissue-specific metabolic environments.
|
|
|
27.11.2019 by Michael Potente
Max Armbruster was awarded the Otto-Hess scholarship by the German Cardiac Society (DGK). Max received this competitive award for his exciting project on the metabolic regulation of endothelial cells. The program aims to support young MD students that perform outstanding research in clinical and basic cardiovascular sciences. The stipend will allow him to focus his efforts on his laboratory work and will be an incentive to pursue a career in academic medicine. He will present a poster of his research findings at an upcoming DGK conference.
|
 ☰
☰